Samantha M. Sawicki, MD, PhD; Cristian Hernandez, BSc; Neda Laiteerapong, MD, MS; and Erin K. Zahradnik, MD
Published: September 18, 2023
ABSTRACT
Objective: Neuropsychiatric symptoms (NPS) of dementia represent a large driver of health care costs, caregiver burden, and institutionalization of people with dementia. Management options are limited, and antipsychotics are often used, although they carry a significant side effect profile. One novel option is tetrahydrocannabinol (THC); however, in the US, to obtain THC for patients with dementia, caregivers have to go to a commercial dispensary. We evaluated the effectiveness of dispensary-obtained THC for patients with dementia and NPS.
Methods: Two independent reviewers reviewed charts of patients with diagnosed dementia (N = 50) seen in geriatric psychiatry between 2017 and 2021 for whom dispensary-obtained THC was recommended. The primary outcome was effectiveness in treating NPS; secondary outcomes were the proportion of caregivers who obtained and administered THC (uptake), post-THC antipsychotic use, and adverse reactions leading to treatment discontinuation.
Results: Caregiver uptake of dispensary-obtained THC was high (38/50, 76%). The majority of patients (30/38, 79%) who took THC had an improvement in NPS according to their caregivers. THC was recommended most often for the NPS of agitation, aggression, irritability, lability, anxiety, and insomnia. Among the 20 patients who were taking antipsychotics at baseline and took THC, over half (12/20, 60%) were able to decrease or discontinue the antipsychotic. Adverse reactions to THC included dizziness, worsening of agitation, and worsening of paranoia; two caregivers of patients who took THC reported adverse reactions that led to treatment discontinuation.
Conclusions: Our results suggest that dispensary-obtained THC can be effective in managing a subset of NPS in patients with dementia and may decrease the requirement for antipsychotics.
J Clin Psychiatry 2023;84(6):23m14791
Author affiliations are listed at the end of this article.
Currently, there are over 55 million people living with dementia worldwide, with nearly 10 million new cases diagnosed annually.1 Dementia is projected to affect 13.9 million Americans by the year 2060 and is becoming a leading cause of morbidity and mortality in the US.2 This disease incurs an estimated $159 to 215 billion in societal costs annually, similar to the financial burden of heart disease and cancer.3 Neuropsychiatric symptoms (NPS), including apathy, sleep disturbance, depression, anxiety, and appetite problems, represent a major source of morbidity and costs in people with dementia.4 NPS affect the majority of people with dementia at some point in their disease5 and are a major driver of adverse patient outcomes including institutionalization, morbidity and mortality, and significant caregiver stress and financial strain.6,–,9
NPS in dementia are well-known to be challenging to manage, and available treatments carry a significant side effect profile. Nonpharmacologic strategies, such as environmental modification, are considered first-line interventions for NPS10; however, nonpharmacologic strategies are frequently insufficient or logistically difficult. As a result, antipsychotic medications are often used,11 although they carry an increased risk of myocardial infarction, hip fractures, extrapyramidal symptoms, and all-cause mortality.12,13 Several professional societies have urged caution in using antipsychotics to treat NPS of dementia, highlighting the need for alternative methods to manage these symptoms.8,14–16 Selective serotonin reuptake inhibitors (SSRIs) are increasingly being used and studied to treat agitation in dementia and have been found to be effective, but carry risks including prolonged corrected QT interval (QTc) and worsening of cognition.17 Studies of additional agents are ongoing.18
One promising class of agents to manage NPS is that of cannabinoids. This drug class includes agonists of the central nervous system CB1 receptor,19 which mediates neurologic functions, including nociception, cognition, motor activity, and mood.20 A recent meta-analysis21 of 9 randomized controlled trials of cannabinoids for NPS found a significant effect of reduction in symptoms (particularly agitation, aggression, and resistance to care) with no major adverse events, but rated the overall study qualities as low.
Notably, all studies of the cannabinoid tetrahydrocannabinol (THC) for NPS in dementia were conducted outside of the US. No previous study has examined the use of THC for the treatment of NPS in the US, where the regulatory environment is different. In the US, on a federal level, cannabis is a Schedule I drug and cannot be directly prescribed by a physician. As a result, if physicians in the US would like to recommend THC to a patient, they must advise the patient or their caregiver to obtain medical cannabis, which contains THC, from a commercial dispensary.
Dispensary-obtained THC via medical cannabis has been used as a treatment option for geriatric psychiatry patients at our institution since the US legalization of medical cannabis in 2014. In this retrospective cohort study, we examine the use of dispensary-obtained THC as a treatment for NPS in patients with dementia in a geriatric psychiatry practice. We report the first real-world study of the use of dispensary-obtained THC for the treatment of NPS in dementia.
METHODS
Study Design and Data Source
We conducted a retrospective cohort study of patients with previously diagnosed dementia seen in an outpatient geriatric psychiatry clinic at the University of Chicago South Shore Senior Center from January 2017 through December 2021. The South Shore Senior Center is a geriatric medicine practice of 7 physicians, 3 advanced nurse practitioners, 2 geriatric fellows, and 2 social workers. Collocated in this clinic is a geriatric psychiatry practice, which is staffed by 1 geriatric psychiatrist who sees approximately 250 patients per year.
Electronic health record (EHR) data for eligible patients were provided by the University of Chicago Center for Research Informatics via its Clinical Research Data Warehouse (CRDW). Two reviewers (S.M.S. and C.H.) independently reviewed each eligible patient’s chart. Data were collected in REDCap.22 Study approval was obtained from the University of Chicago Biological Sciences Division Institutional Review Board.
Study Population
All patients with a visit diagnosis of dementia seen in the geriatric psychiatry outpatient clinic during the 4-year study period were screened for inclusion. Patients were included if THC was recommended for NPS during the study period. In general, the University of Chicago geriatric psychiatrist (E.K.Z.) recommends THC in an edible formulation to patients with moderate to advanced dementia who present with unmanaged NPS. Patients’ caregivers are advised to obtain THC from a dispensary and to begin by administering 2.5 mg of THC between 1 and 5 pm daily, because many NPS emerge in the evening. Low doses are used initially to avoid adverse reactions in cannabinoid-naive patients. The usual form of THC is a quartered 10-mg gummy.
Primary Outcome
As our primary outcome, we analyzed the effectiveness of dispensary-obtained THC for treating NPS of dementia. Two independent reviewers examined provider notes to determine caregiver-reported effectiveness, which was classified as “effective,” “not effective,” or “not able to determine” if documentation was incomplete. Also, reviewers recorded provider documentation about effectiveness verbatim from the medical chart to qualitatively analyze these comments. Specifically, THC was classified as effective in treating NPS if there was specific provider documentation regarding the effect of THC on NPS that included the following words or phrases: “doing well,” “working well,” “doing better,” “well-controlled,” “effective,” “helping/helped/helpful,” “improved,” or “beneficial.” THC was classified as not effective if there was provider documentation regarding the effect of THC on NPS that included the following: “no benefit,” “paradoxical response,” “not sure it is doing anything,” or “lasts a short period.”
Secondary Outcomes
Secondary outcomes included the frequency of caregiver uptake of the recommendation to obtain THC from a dispensary and administer it to the patient, patterns of antipsychotic use after THC, and adverse effects of THC. We also assessed via chart review whether patients went on to pursue medical cannabis cards.
Covariates
Type of dementia was determined by the initial visit encounter documentation. If type of dementia was unspecified in the initial visit, other visit encounters in primary care and neurology were reviewed to identify whether a more specific type of dementia had been previously diagnosed. NPS were categorized according to the Neuropsychiatric Inventory (NPI) categories.23 The use patterns of antipsychotics were determined by reviewing provider notes and prescription history.
Analysis
Basic descriptive statistical analyses including calculation of mean and standard deviation were performed in Microsoft Excel (2018, version 16.16.27). Additionally, a qualitative template analysis was conducted on the documentation related to THC effectiveness.
RESULTS
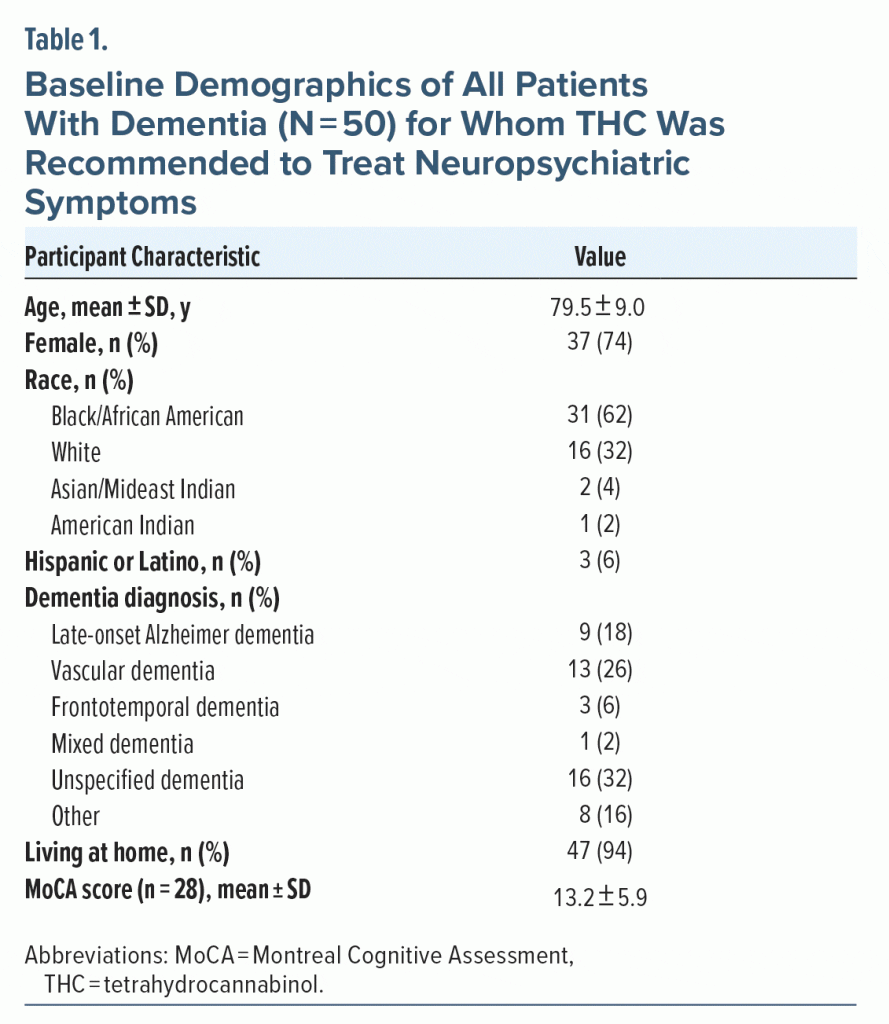
A total of 249 patients with an encounter diagnosis of dementia had a visit in the geriatric psychiatry clinic between January 2017 and December 2021. Of these, 50 patients were eligible for inclusion; 62% (n = 31) were Non-Hispanic Black/African American, and 74% (n = 37) were female (see Table 1). The mean (SD) age was 79.5 (9.0) years. The most common dementia diagnoses were unspecified dementia (32%, n = 16) and vascular dementia (26%, n = 13). Most patients (92%, n = 46) had “with behavioral disturbance” as part of their visit diagnosis. Of the patients with documented Montreal Cognitive Assessment (MoCA)24 scores (n = 28), the mean MoCA score was 13/30, reflecting moderate cognitive impairment (SD = 6; range, 3–25). All patients had a family member or caregiver present at the visit, and 47 patients (94%) were residing at home.
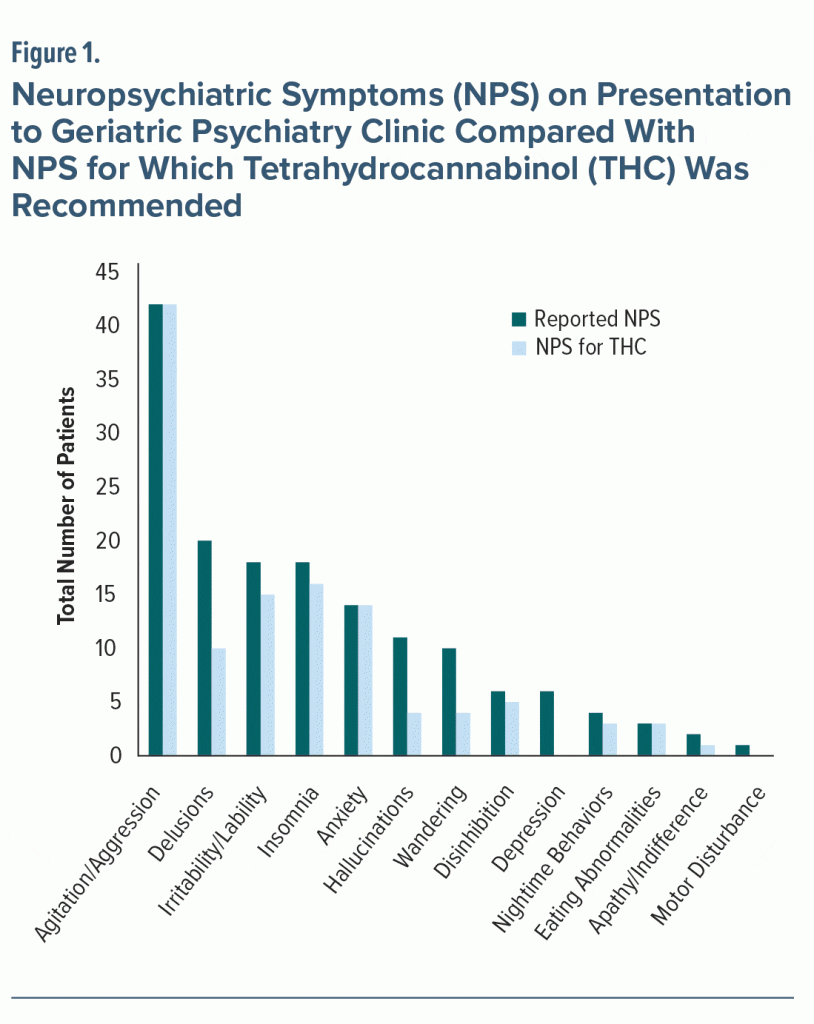
The distribution of NPS noted in the patients’ psychiatry visits is shown in Figure 1. The most common NPS on presentation were agitation/aggression (84%, n = 42), delusions (40%, n = 20), insomnia (36%, n = 18), and irritability/lability (36%, n = 18). The primary NPS for which THC was recommended were agitation/aggression (84%, n = 42), insomnia (32%, n = 16), irritability/lability (30%, n = 15), and anxiety (28%, n = 14) (Figure 1). Most patients (42/50) reported the presence of more than one neuropsychiatric symptom.
Following the THC recommendation, 38 patients (76%) started taking THC, and most (79%, n = 30) of those who started THC used it throughout the study period. Among the 12 patients who did not start THC, 4 patients resided in a neighboring state with legislation prohibiting caregivers from obtaining THC, and 1 caregiver stated cost was a prohibitive factor in obtaining THC. Over half of the patients who used THC (21/38) used a dose of 2.5 mg, most often in the form of a quartered gummy. Five patients required a higher dose of 5 mg. One patient used a range from 2.5 to 5 mg. Three patients used a cannabidiol (CBD) and THC combination product, the dosing of which could not be determined by chart review. Eight patients used THC at a dose that could not be determined by chart review. We could confirm by chart review that 22 of 38 patients who used THC took THC daily in the afternoon or evening. Two patients used THC twice a day, and 1 patient used it in the morning. One patient used it once daily as needed. The timing for the remaining patients who used THC could not be determined by chart review.
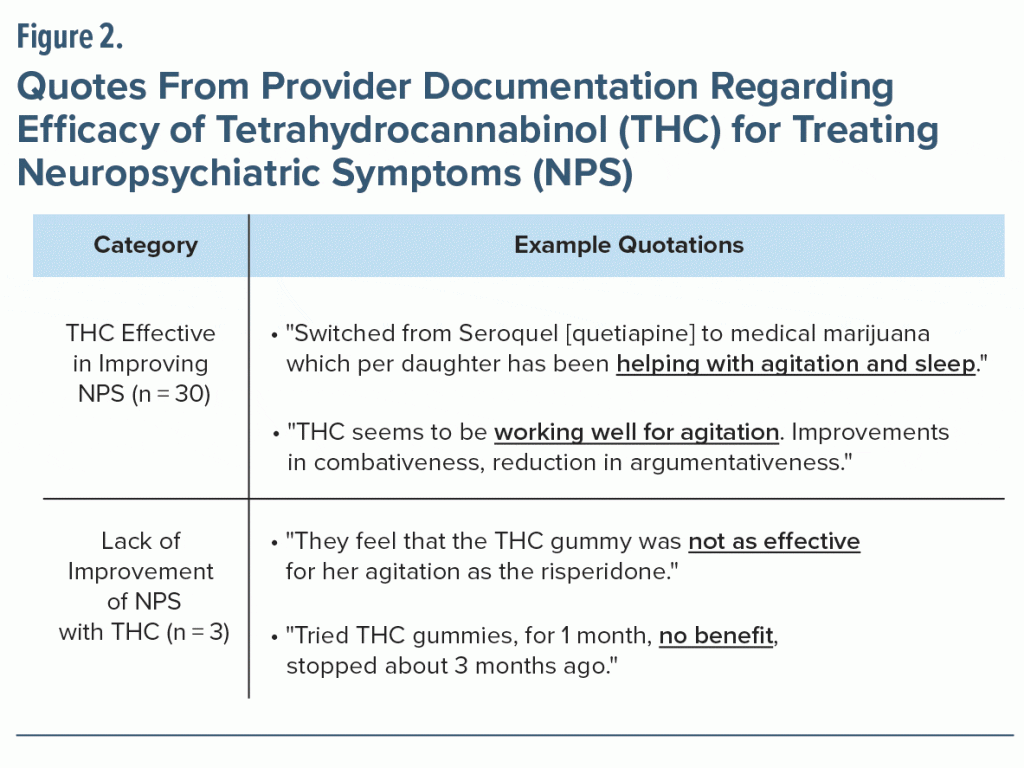
We found that 30 of 38 caregivers (79%) reported that THC was effective in improving NPS; only 3 caregivers described a lack of improvement with THC usage. Figure 2 displays examples of caregiver quotes pulled from patients’ charts. Provider’s documentation of one caregiver’s comments about efficacy stated that “THC seems to be working well for agitation, improvements in combativeness, [and] reduction in argumentativeness.” Documentation about another caregiver who noted THC was not effective stated, “They feel that the THC gummy was not as effective for her agitation as the risperidone.” Fewer than half of patients (13/50) requested assistance in pursuing a medical cannabis card.
Among dementia subtypes, THC treatment was effective in 100% of patients (8/8) with late-onset Alzheimer’s dementia, 90% of patients (9/10) with vascular dementia, and 83% of patients (10/12) with unspecified dementia. The 2 patients with early-onset Alzheimer’s disease and 1 patient with frontotemporal dementia did not find THC effective for their NPS.
Five patients (10%) reported an adverse reaction from THC. These reactions included dizziness, worsening of agitation, and worsening of paranoia. One caregiver reported that the patient seemed “zoned out of it.” Two of the 5 patients who experienced an adverse drug event discontinued taking THC.
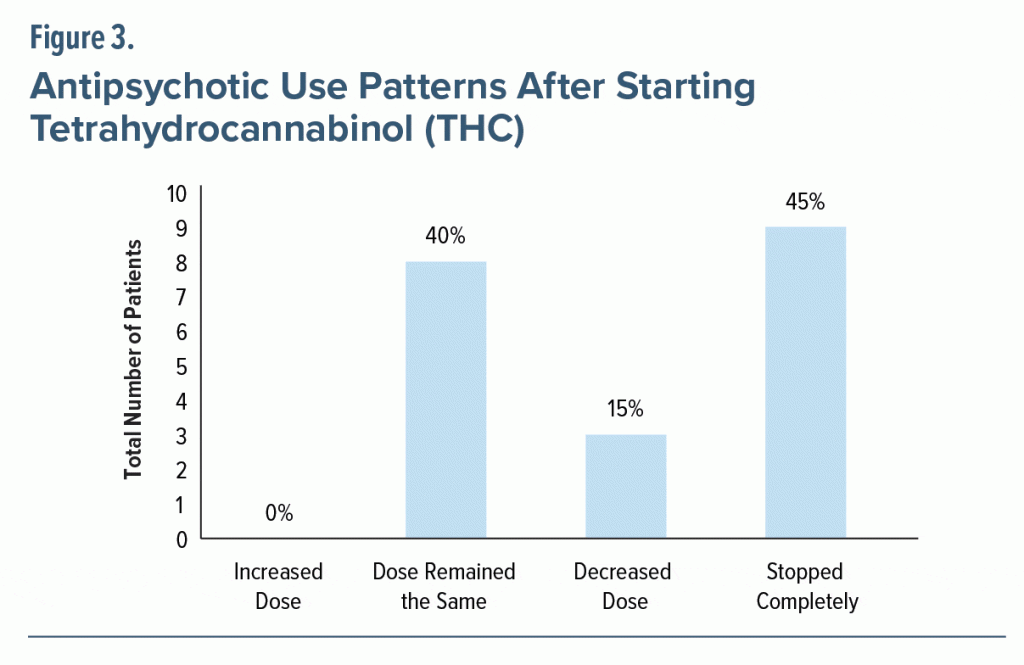
In regard to antipsychotic use, about half (n = 20, 53%) of patients who began THC were taking antipsychotics at baseline. The most common drugs were quetiapine, risperidone, and olanzapine. As seen in Figure 3, after THC was initiated, over half of patients (n = 12, 60%) were able to decrease or discontinue their dose of antipsychotics. No patients required an increase in their antipsychotic use after they began treatment with THC.
DISCUSSION
In this first US study describing real-world evidence for THC obtained from a dispensary for treating NPS in dementia, we found that dispensary-obtained THC was effective for most patients with dementia. We found that most caregivers were able to obtain and administer THC, and there were few discontinuations due to adverse reactions. Also, over half of patients who were taking antipsychotics prior to THC initiation were able to decrease or discontinue antipsychotics after starting THC.
On the basis of subjective reports from caregivers, THC was effective in treating NPS of dementia in the majority of patients who used it. Agitation, aggression, irritability, lability, anxiety, and insomnia were the most common NPS presentations for which THC was recommended. Agitation and aggression confer a particularly high burden on caregivers and are a common reason for antipsychotic initiation in this patient population.6 Our results provide preliminary evidence that THC may be an alternative treatment for these symptoms in particular.
No major adverse reactions were found in our cohort, which was encouraging. There is the potential for adverse reactions because of the presence of CB1 and CB2 receptors in numerous organ systems, including the cardiovascular system, the immune system, and the central nervous system.25 Notably, patients who used THC did not develop psychosis or seizures. Previous studies of the use of cannabinoids in patients with dementia have also found a positive safety profile, with most adverse drug effects categorized as mild.19,21 Larger real-world studies of THC in patients with dementia and NPS would inform estimates of rates of adverse reactions.
We also found that most caregivers were able to follow through with the recommendation to obtain THC from a dispensary and give it to the patient. This was a somewhat unexpected finding because we predicted that the hurdle of obtaining the product from a dispensary would be a deterrent. However, the majority of patients ultimately used the product and continued it throughout our study review period. One factor that may have facilitated this uptake is the urban nature of the medical practice and the numerous dispensaries in the surrounding zip codes.
Our findings are encouraging in that the real-world practice of obtaining THC from a dispensary and administering it to patients is feasible and effective. Many caregivers of patients with dementia do not have safe and effective options for treating NPS, which can be very burdensome and lead to nursing home placement. Geriatricians and psychiatrists are also challenged when treating NPS because commonly used treatment options such as antipsychotics carry significant side effects in this population. The possibility of sparing patients exposure to antipsychotics is compelling for caregivers, clinicians, and health care systems given the known serious risks associated with long-term use.
Our study was a retrospective chart review analysis, which poses several limitations. Patients were not randomized, and therefore there may be selection bias in the patients for whom THC was recommended. Also, the geriatric psychiatry clinic did not use a neuropsychiatric assessment tool in clinical practice, which would have standardized measurement of changes in NPS. Additionally, because the THC was obtained through dispensaries and the THC dose was a fraction of a gummy, the actual dose of THC administered to patients was non-standard and could have differed, leading to different treatment effectiveness and side effects. The side effect profile of the medication was not formally assessed and is limited by the retrospective nature of chart review.
It is also important to mention the current regulatory environment around cannabis legality in the United States. Cannabis for medical use is currently legal in 38 states. In Illinois, small amounts of cannabis can be purchased legally for recreational purposes. Therefore, it is not surprising that fewer than half of caregivers obtained medical cannabis cards. However, on a federal level, it is a Schedule I drug and is not covered by any insurance prescription plan. It is generally not available currently in acute and post-acute care settings or in many long-term care facilities. However, this landscape is changing, and there have been recent initiatives to allow facility-dwelling patients to participate in state-approved cannabis programs.26
CONCLUSIONS
Here we report the first study of dispensary-obtained THC for the treatment of NPS in dementia. Our results suggest that THC may be an effective treatment for agitation, aggression, irritability, lability, anxiety, and insomnia. We found a high degree of caregiver follow-through on the recommendation to obtain and administer THC. Over half of patients on treatment with antipsychotics were able to decrease or discontinue antipsychotics once they began taking THC. This early evidence warrants further controlled studies of THC for the treatment of NPS in dementia. In the rapidly changing regulatory landscape of cannabis for medical purposes, it is urgent that we have high-quality evidence to guide clinicians on effective and safe practices.
Article Information
Published Online: September 18, 2023. https://doi.org/10.4088/JCP.23m14791
© 2023 Physicians Postgraduate Press, Inc.
Submitted: January 9, 2023; accepted June 27, 2023.
To Cite: Sawicki SM, Hernandez C, Laiteerapong N, et al. The use of dispensary-obtained tetrahydrocannabinol as a treatment for neuropsychiatric symptoms of dementia. J Clin Psychiatry. 2023;84(6):23m14791.
Author Affiliations: University of Chicago Medical Center, Department of Medicine, Chicago, Illinois (Sawicki, Hernandez, Laiteerapong, Zahradnik); University of Chicago Medical Center, Department of Psychiatry and Behavioral Neuroscience, Chicago, Illinois (Zahradnik).
Corresponding Author: Erin K. Zahradnik, MD, Department of Psychiatry and Behavioral Neuroscience, 5841 S Maryland Ave, Chicago, IL 60637 (ezahradnik1@uchicagomedicine.org).
Dr Sawicki and Mr Hernandez are co-first authors.
Author Contributions: Drs Sawicki, Laiteerapong, and Zahradnik and Mr Hernandez contributed to the conception and design of the work, drafting and revising the work for important intellectual content, and final approval of the version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Relevant Financial Relationships: The authors report no conflicts with any product mentioned or concept discussed in this article.
Funding/Support: We utilized REDCap software, which receives support from National Institutes of Health (NIH) CTSA UL1 TR000430. This project was also supported by funding from National Institute on Aging (NIA) Grant #5T35AG029795-15.
Role of the Funders/Sponsors: The funder had no role in the conduct and publication of this study.
Previous Presentation: Poster presented at the AGS Annual Meeting; May 2022; Orlando, Florida.
CLINICAL POINTS
- Neuropsychiatric symptoms are nearly ubiquitous in dementia; however, current treatment options are limited by significant side effect profiles.
- Dispensary-obtained tetrahydrocannabinol may be an effective alternative treatment options for patients with dementia presenting with neuropsychiatric symptoms, particularly agitation, anxiety, irritability, and insomnia.
Editor’s Note: We encourage authors to submit papers for consideration as a part of our Focus on Geriatric Psychiatry section. Please contact Jordan F. Karp, MD, at jkarp@psychiatrist.com, or Gary W. Small, MD, at gsmall@psychiatrist.com.
Quick Links: Dementia
REFERENCES
DOWNLOAD CHECKED REFERENCES
CHECK ALL
UNCHECK ALL
1. World Health Organization. Global action plan on the public health response to dementia: 2017–2025. WHO website. https://apps.who.int/iris/bitstream/handle/10665/259615/9789241513487-eng.pdf?sequence=1. 2017. Accessed February 2, 2023.
2. Matthews KA, Xu W, Gaglioti AH, et al. Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015-2060) in adults aged ≥65 years. Alzheimers Dement. 2019;15(1):17–24. PubMed CrossRef Show Abstract
3. Hurd MD, Martorell P, Delavande A, et al. Monetary costs of dementia in the United States. N Engl J Med. 2013;368(14):1326–1334. PubMed CrossRef Show Abstract
4. Radue R, Walaszek A, Asthana S. Chapter 24 - Neuropsychiatric symptoms in dementia. Handbook of Clinical Neurology. Dekosky ST, Asthana S, eds. Vol 167, Geriatric Neurology. Elsevier, 2019;437–454. https://www.sciencedirect.com/science/article/pii/B9780128047668000248. Accessed September 13, 2021.
5. Lyketsos CG, Lopez O, Jones B, et al. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA. 2002;288(12):1475–1483. PubMed CrossRef Show Abstract
6. Okura T, Langa KM. Caregiver burden and neuropsychiatric symptoms in older adults with cognitive impairment: the Aging, Demographics, and Memory Study (ADAMS). Alzheimer Dis Assoc Disord. 2011;25(2):116–121. PubMed CrossRef Show Abstract
7. Phan SV, Osae S, Morgan JC, et al. Neuropsychiatric Symptoms in Dementia: Considerations for Pharmacotherapy in the USA. Drugs R D. 2019;19(2):93–115. PubMed CrossRef Show Abstract
8. Banerjee S, Murray J, Foley B, et al. Predictors of institutionalisation in people with dementia. J Neurol Neurosurg Psychiatry. 2003;74(9):1315–1316. PubMed CrossRef Show Abstract
9. Okura T, Plassman BL, Steffens DC, et al. Neuropsychiatric symptoms and the risk of institutionalization and death: the aging, demographics, and memory study. J Am Geriatr Soc. 2011;59(3):473–481. PubMed CrossRef Show Abstract
10. Kales HC, Gitlin LN, Lyketsos CG; Detroit Expert Panel on Assessment and Management of Neuropsychiatric Symptoms of Dementia. Management of neuropsychiatric symptoms of dementia in clinical settings: recommendations from a multidisciplinary expert panel. J Am Geriatr Soc. 2014;62(4):762–769. PubMed CrossRef Show Abstract
11. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–446. PubMed CrossRef Show Abstract
12. Farlow MR, Shamliyan TA. Benefits and harms of atypical antipsychotics for agitation in adults with dementia. Eur Neuropsychopharmacol. 2017;27(3):217–231. PubMed CrossRef Show Abstract
13. Meeks TW, Jeste DV. Beyond the black box: what is the role for antipsychotics in dementia? Curr Psychiatr. 2008;7(6):50–65. PubMed Show Abstract
14. Schneider LS, Tariot PN, Dagerman KS, et al; CATIE-AD Study Group. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med. 2006;355(15):1525–1538. PubMed CrossRef Show Abstract
15. Reus VI, Fochtmann LJ, Eyler AE, et al. The American Psychiatric Association Practice Guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543–546. PubMed CrossRef Show Abstract
16. American Geriatrics Society. Choosing Wisely website. https://www.choosingwisely.org/societies/american-geriatrics-society/. Accessed February 24, 2015, November 5, 2022.
17. Porsteinsson AP, Drye LT, Pollock BG, et al; CitAD Research Group. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA. 2014;311(7):682–691. PubMed CrossRef Show Abstract
18. Ehrhardt S, Porsteinsson AP, Munro CA, et al; S-CitAD Research Group. Escitalopram for agitation in Alzheimer’s disease (S-CitAD): methods and design of an investigator-initiated, randomized, controlled, multicenter clinical trial. Alzheimers Dement. 2019;15(11):1427–1436. PubMed CrossRef Show Abstract
19. Hillen JB, Soulsby N, Alderman C, et al. Safety and effectiveness of cannabinoids for the treatment of neuropsychiatric symptoms in dementia: a systematic review. Ther Adv Drug Saf. 2019;10:2042098619846993. PubMed CrossRef Show Abstract
20. Walther S, Halpern M. Cannabinoids and dementia: a review of clinical and preclinical data. Pharmaceuticals (Basel). 2010;3(8):2689–2708. PubMed CrossRef Show Abstract
21. Bahji A, Meyyappan AC, Hawken ER. Cannabinoids for the neuropsychiatric symptoms of dementia: a systematic review and meta-analysis. Can J Psychiatry. 2020;65(6):365–376. PubMed CrossRef Show Abstract
22. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. PubMed CrossRef Show Abstract
23. Cummings JL, Mega M, Gray K, et al. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–2314. PubMed CrossRef Show Abstract
24. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. PubMed CrossRef Show Abstract
25. Sachs J, McGlade E, Yurgelun-Todd D. Safety and toxicology of cannabinoids. Neurotherapeutics. 2015;12(4):735–746. PubMed CrossRef Show Abstract
26. Palace ZJ, Reingold DA. Medical cannabis in the skilled nursing facility: a novel approach to improving symptom management and quality of life. J Am Med Dir Assoc. 2019;20(1):94–98. PubMed CrossRef Show Abstract